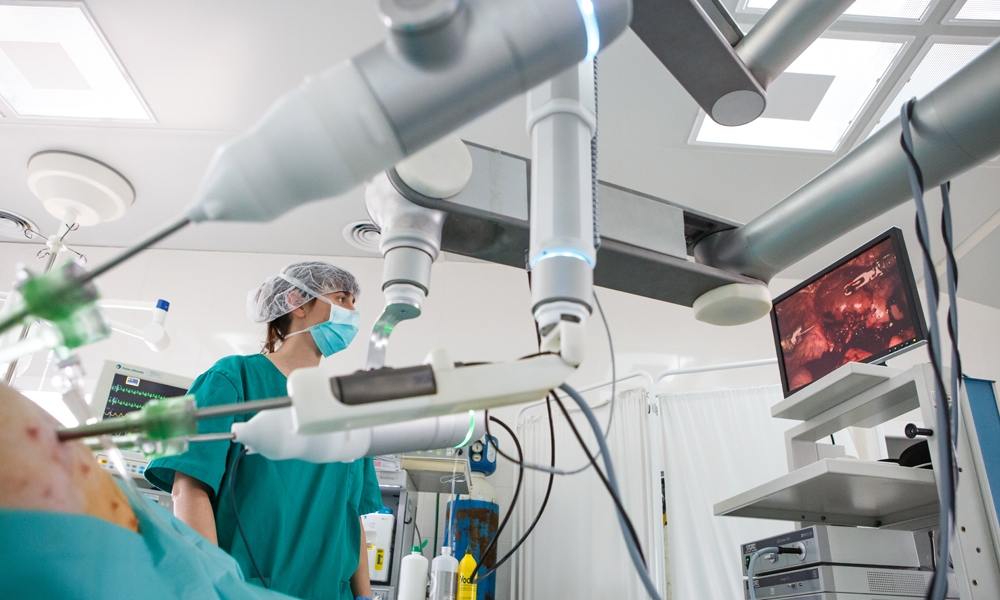
The new Bitrack system for minimally invasive surgery developed by Rob Surgical has successfully completed its technical validation on experimental models and is preparing for European authorisation.
Bitrack is the modular, flexible and more economical alternative to the American Da Vinci robot installed in many leading hospitals around the world.
The trials using the final prototype were conducted in the new experimental operating theatre at Specipig. The trials involved surgeons specialising in surgical robotics with knowledge of the Da Vinci system, including Dr Javier Magriña, Mayo Clinic surgeon. The technical management of the project was the responsibility of Prof Josep Amat, academic at the Technical University of Catalonia and advisor of robotics at the Research Centre for Biomedical Engineering (CREB-UPC).
“The global challenge of robotic surgery is to improve efficiency, in other words, without losing any of the effectiveness of the current systems make it possible to acquire robots at a lower cost to meet certain technical demands of the medical community. We set out to put robotic surgery within the reach of most hospitals”, explains Jaume Amat, CEO of Rob Surgical.
Robotic surgery is perceived as a source of added value for the patient, surgeon and hospital, enabling complex operations to be carried out with great precision. The market is estimated to be worth 5 billion euros per year, with an annual growth rate of 30% over the last five years. Currently 99% of the surgical robotics market share is held by the American company Intuitive Surgical.
An investment round is opened
“We have completed the R+D phase led by an internationally renowned scientific and technical team. We now have a functional prototype protected by international patents which meets real needs of the market. The remaining steps for bringing it to the market and the business model are clear”, Jaume Amat tells us.
In the next few months, an investment round will be opened to cover the last phase of the project, and contacts with medical technology companies about licensing will continue.
In the media:
- Rob Surgical prepara una ronda de 3 millones (Expansión)
- Bitrack está listo para democratizar la cirugía robótica (El Mundo)
- El robot quirúrgico Bitrack concluye la validación técnica con éxito (Immedicohospitalario.es)
- Nuevo robot para cirugía mínimamente invasiva alternativo al Da Vinci (Consalud.es)
- The Bitrack surgical robot has successfully completed its technical validation (Firstwordmedtech.com)