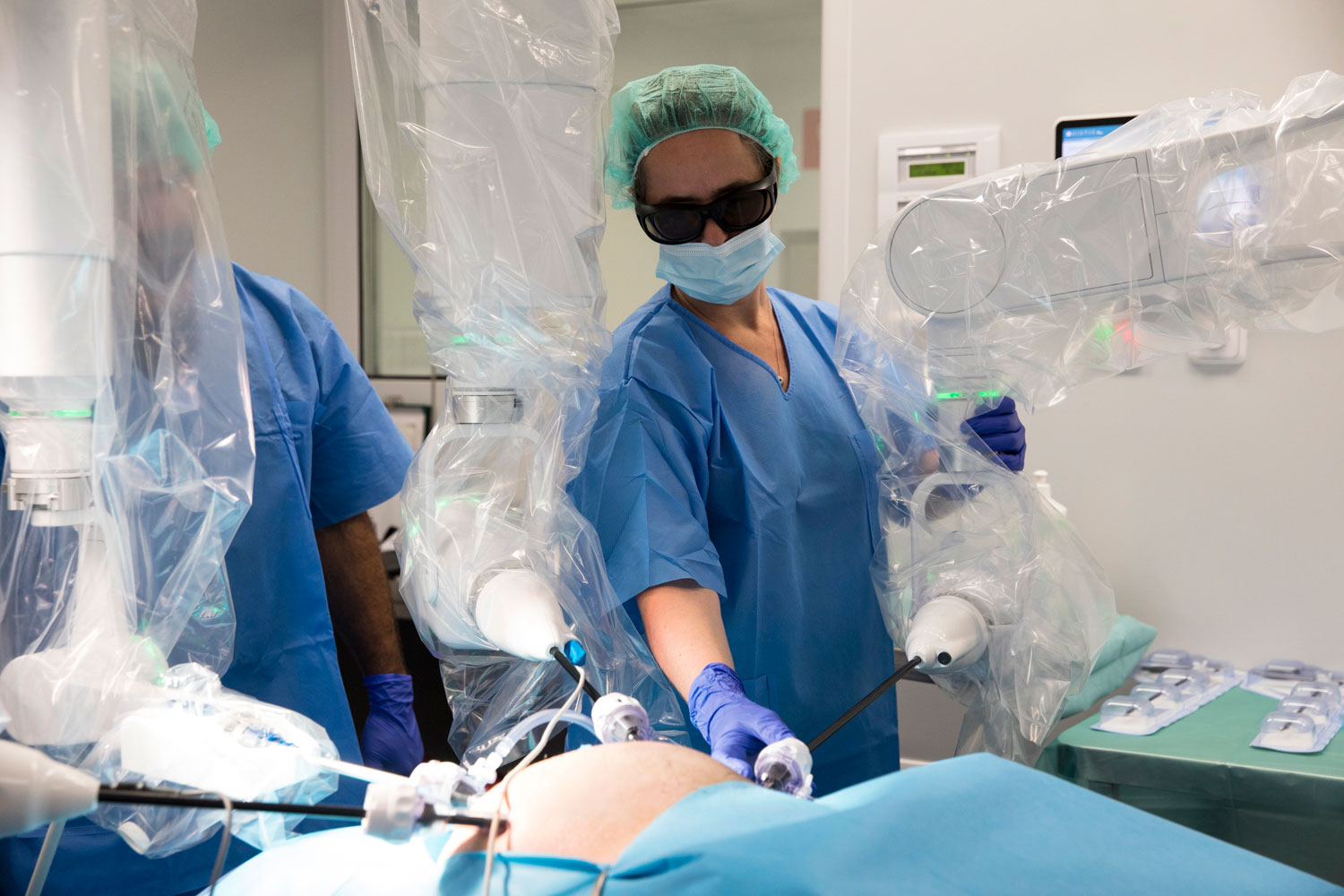
Rob Surgical is in the final approval phase for its surgical robot. It is expected to receive Europe wide certification in mid 2024 and in the USA in mid 2025. Bitrack System, Rob Surgical robot, is the only 4-arms, open platform, on-demand and accessible robotic solution in the market aimed at giving surgeons open access to patients; for hospitals lower cost and better versatility; and for patients access to benefits of robotic surgeries that are currently only for 2% of cases.
The precision surgery company announced in June that it had successfully performed the first surgeries with its surgical robot in collaboration with Hospital Clínic, the prelude to its certification. Rob Surgical will be the 8th company to reach CE marking.
Rob Surgical has just incorporated Todd Usen to lead its growth plan. Mr. Usen is a highly respected healthcare executive with extensive experience in scaling and corporate governance. Todd has previously worked as Chairman, Non-Executive Director and CEO leading organizations from technology concept to FDA and CE mark clearance over the last 25 years.
With these milestones achieved, the company confirms its marketing and production plan for 2025, which include the manufacture and installation of 50 units of its surgical platform in hospitals throughout Europe, as well as intensive use of the robot in over 500 surgeries in the urological field, for which the company will have validated the full scope of the interventions. The company, which has raised more than €20 million through different investment rounds, is exploring new partnerships with key partners to achieve its goals by 2025.